UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|||
|
|
|
|
|
|
|||
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification Number) |
|||
(
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
|
|
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR § 230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR § 240.12b-2).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
The slides attached as Exhibit 99.1 to this Current Report contain certain additional information related to the clinical data results discussed in Item 8.01 below. Crinetics Pharmaceuticals, Inc. (the “Company” or “Crinetics”) intends to present the slides during a conference call and live webcast with the investment community on September 15, 2021, at 4:30 p.m. Eastern Time.
The information contained in this Item 7.01, including in Exhibit 99.1 hereto, is being “furnished” and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, is not subject to the liabilities of that section and is not deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except as shall be expressly set forth by specific reference in such a filing.
Item 8.01 Other Events.
On September 15, 2021, Crinetics announced positive preliminary findings from the single ascending dose (SAD) portion of a first-in-human Phase 1 clinical study with CRN04777 demonstrating pharmacologic proof-of-concept for CRN0477, its investigational, oral, nonpeptide somatostatin receptor type 5 (SST5) agonist that is being developed for the treatment of congenital hyperinsulinism (HI).
The pharmacologic effects of CRN04777 were evaluated using two distinct methods. First, oral administration of CRN04777 showed rapid dose-dependent suppression of insulin secretion in response to an intravenous bolus of glucose in an Intravenous Glucose Tolerance Test, or IVGTT. In a second method, oral administration of CRN04777 rapidly eliminated the need for IV glucose support in individuals who were administered a sulfonylurea, a class of drugs that induces insulin secretion analogous to the most common genetic defect in congenital HI patients. The reductions in insulin secretion and resulting changes in plasma glucose in these pharmacologic evaluations suggest that CRN04777 binds and activates pancreatic beta-cell SST5 to inhibit insulin secretion, as designed. CRN04777 was well tolerated in the healthy volunteers who enrolled in these SAD cohorts and all adverse events were considered mild or moderate.
Forward-Looking Statements
Crinetics cautions you that statements contained in this current report regarding matters that are not historical facts are forward-looking statements. These statements are based on the Company’s current beliefs and expectations. Such forward-looking statements include, but are not limited to, statements regarding the potential benefits of CRN04777 for patients with congenital hyperinsulinism. The inclusion of forward-looking statements should not be regarded as a representation by Crinetics that any of its plans will be achieved. Actual results may differ from those set forth in this current report due to the risks and uncertainties inherent in Crinetics’ business, including, without limitation: preliminary data that we report may change following a more comprehensive review of the data related to the clinical trials and such data may not accurately reflect the complete results of a clinical trial, and the FDA and other regulatory authorities may not agree with our interpretation of such results; advancement of CRN04777 into later stage trials is dependent on and subject to the receipt of further feedback from the FDA; we may not be able to obtain, maintain and enforce our patents and other intellectual property rights, and it may be prohibitively difficult or costly to protect such rights; the COVID-19 pandemic may disrupt Crinetics’ business and that of the third parties on which it depends, including delaying or otherwise disrupting its clinical trials and preclinical studies, manufacturing and supply chain, or impairing employee productivity; the Company’s dependence on third parties in connection with product manufacturing, research and preclinical and clinical testing; the success of Crinetics’ clinical trials and nonclinical studies for paltusotine, CRN04894, CRN04777, and its other product candidates; regulatory developments in the United States and foreign countries; unexpected adverse side effects or inadequate efficacy of the Company’s product candidates that may limit their development, regulatory approval and/or commercialization; Crinetics may use its capital resources sooner than it expects; and other risks described under the heading “Risk Factors” in documents the Company files from time to time with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Crinetics undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit No |
|
Description |
|
|
|
|
|
|
|
99.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
Date: September 15, 2021 |
|
Crinetics Pharmaceuticals, Inc. |
|
|
|
|
|
|
|
/s/ R. Scott Struthers, Ph.D. |
|
|
|
R. Scott Struthers, Ph.D. |
|
|
|
President and Chief Executive Officer (Principal Executive Officer) |
3

CRN04777: First in Human single ascending dose (SAD) Preliminary Results September 15, 2021 Exhibit 99.1

This presentation contains forward-looking statements. Crinetics cautions you that statements contained in this presentation regarding matters that are not historical facts are forward-looking statements. These statements are based on the company’s current beliefs and expectations. Such forward-looking statements include, but are not limited to, statements regarding: the potential benefits of CRN04777 for patients with congenital hyperinsulinism and plans to advance CRN04777, paltusotine and CRN04894 into additional clinical trials and the timing thereof. The inclusion of forward-looking statements should not be regarded as a representation by Crinetics that any of its plans will be achieved. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in Crinetics’ business, including, without limitation: preliminary data that we report may change following a more comprehensive review of the data related to the clinical trials and such data may not accurately reflect the complete results of a clinical trial, and the FDA and other regulatory authorities may not agree with our interpretation of such results; advancement of CRN04777 into later stage trials is dependent on and subject to the receipt of further feedback from the FDA; we may not be able to obtain, maintain and enforce our patents and other intellectual property rights, and it may be prohibitively difficult or costly to protect such rights; the COVID-19 pandemic may disrupt Crinetics’ business and that of the third parties on which it depends, including delaying or otherwise disrupting its clinical trials and preclinical studies, manufacturing and supply chain, or impairing employee productivity; the company’s dependence on third parties in connection with product manufacturing, research and preclinical and clinical testing; the success of Crinetics’ clinical trials and nonclinical studies for paltusotine, CRN04894, CRN04777, and its other product candidates; regulatory developments in the United States and foreign countries; unexpected adverse side effects or inadequate efficacy of the company’s product candidates that may limit their development, regulatory approval and/or commercialization; Crinetics may use its capital resources sooner than it expects; and other risks described under the heading “Risk Factors” in documents the company files from time to time with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Crinetics undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and, except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Safe Harbor Statement CRINETICS PHARMACEUTICALS | 2

Phase 1 Pharmacologic Proof-of-Concept for CRN04777 in Healthy Volunteers CRINETICS PHARMACEUTICALS | 3

Crinetics’ Endocrine Development Strategy: Hormone Levels from Preclinical to Approval Phase 1 Healthy Volunteers Phase 2/3 Trials (Patients) CRINETICS PHARMACEUTICALS | 4

Congenital Hyperinsulinism (HI) Results in Life Threatening Recurrent Hypoglycemia CRINETICS PHARMACEUTICALS | 5 Congenital HI patients secrete insulin even when blood sugar is low X Beta-cell mutations cause inappropriate insulin secretion Inappropriate insulin causes hypoglycemia Untreated hypoglycemia can result in neurodevelopment disorders and death Early identification and continuous intensive glucose management are critical Current treatment paradigms place high burden of care on families with all too frequent suboptimal outcomes Six Global Centers of Excellence named for treatment of patients with HI Robust global patient advocacy such as Congenital Hyperinsulinism International (www.congenitalhi.org) Congenital HI is a devastating rare disease

Unmet Medical Needs in Congenital HI are Very High CRINETICS PHARMACEUTICALS | 6 Patient & Parent Goals Avoid hypoglycemia and its consequences including neurological damage Safely sleep through the night Avoid pancreatectomy Eliminate feeding tubes Reduce injections and glucose sticks Avoid side effects of diazoxide and other treatments Medical management until HI resolves with age Be a kid not a patient

CRN04777: First-in-class Oral SST5 Agonist with Potential to be Broadly Effective in HI CRINETICS PHARMACEUTICALS | 7 Liver Directed IV glucagon, glucagon rescue pen In development: Glucagon & Analogs (Subcutaneous) Tissues Insulin (b-cells) Glucose Release Glucose Uptake Glucagon (a-cells) Low blood sugar High blood sugar Pancreas Directed to suppress insulin secretion Diazoxide Ineffective in 50%; black box warning Off label use of injectable SST2 agonists Pancreatectomy (complete or partial) In development: GLP-1 antagonist (IV) Designed to inhibit insulin secretion in all HI patients

Somatostatin Receptor SST5 Inhibits Insulin Secretion Downstream of all Known HI Causing Mutations CRINETICS PHARMACEUTICALS | 8 Syndromic hyperinsulinisms (e.g. those associated with Beckwith-Wiedemann syndrome, Sotos syndrome, Kabuki syndrome, and Turner syndrome) may also respond to SST5 agonism SST5 receptor Inappropriate insulin secretion Depolarization Ca2+ channel Glucose GLUT2 ATP/ADP Glycolysis Amino acids Ca2+ KATP channel Glucose sensing Glucose metabolism Hypoglycemia X X X X Amino acid metabolism Pancreas CRN04777 (SST5 Agonist) Inhibition of insulin secretion Prevention of hypoglycemia

CRN04777 is the Only Oral SST5 Agonist in Clinical Development CRN04777 suppressed sulfonylurea (SU)-induced insulin secretion and reversed hypoglycemia in rats CRN04777 is a potent (EC50=0.4 nM) and selective SST5 agonist Sulfonylurea (SU) 30 mg/kg Vehicle SU + 30 mg/kg CRN04777 SU + 10 mg/kg CRN04777 SU + 3 mg/kg CRN04777 CRINETICS PHARMACEUTICALS | 9 hSST1 hSST2 hSST3 hSST4 hSST5

CRN04777 SAD Study Design to Evaluate Pharmacologic Proof-of-Concept Follows Crinetics’ core endocrine strategy of using hormonal biomarkers to drive development CRINETICS PHARMACEUTICALS | 10

PK Results: CRN04777 Showed Oral Bioavailability with Dose-Proportional Exposure 0.5 mg 1 mg 3 mg 9 mg 27 mg 60 mg 120 mg LLOQ Data shown are mean±SEM; LLOQ = lower limit of quantitation All doses n=6; except n=12 for 60 mg which was evaluated in both IVGTT and sulfonylurea challenge *When 120 mg was administered within 30 minutes of a standard adult high fat breakfast, a significant reduction in exposure was observed. Evaluation of pediatric relevant meals pending. CRINETICS PHARMACEUTICALS | 11 Half-life ~40 hours and tmax ~1-2 hours at efficacious doses

CRN04777 SAD Study Designed to Evaluate Pharmacologic Proof-of-Concept Follows Crinetics’ core endocrine strategy of using hormonal biomarkers to drive development Study Goals Evaluate safety [0.5-120 mg] Evaluate pharmacokinetics: oral absorption, dose-proportional exposure, half-life [0.5-120 mg] Evaluate dose response and PK/PD on pre- and post-stimulated glucose and insulin in an IVGTT [0.5-120 mg] Evaluate dose response and PK/PD on reduction/reversal of sulfonylurea-induced insulin secretion (pharmacologic model of disease) [30-60 mg] CRINETICS PHARMACEUTICALS | 12 Healthy volunteers received single oral dose of CRN04777 (n = 8, 6 active/ 2 placebo in each cohort)

CRN04777 Dose-Dependently Suppressed Glucose Stimulated Insulin Secretion Data shown are mean±SEM N=6 CRN04777 treated per dose; N=14 placebo IVGTT=intravenous glucose tolerance test; PBO=placebo CRINETICS PHARMACEUTICALS | 13 CRN04777 dose-dependently reduced insulin secretion stimulated by bolus IV glucose (IVGTT)… PBO 27 mg 60 mg 120 mg …and reduced insulin secretion reduces glucose uptake by tissues resulting in prolonged elevation of plasma glucose

CRN04777 SAD Study Designed to Evaluate Pharmacologic Proof-of-Concept Follows Crinetics’ core endocrine strategy of using hormonal biomarkers to drive development Study Goals Evaluate safety [0.5-120 mg] Evaluate pharmacokinetics: oral absorption, dose-proportional exposure, half-life [0.5-120 mg] Evaluate dose response and PK/PD on pre- and post-stimulated glucose and insulin in an IVGTT [0.5-120 mg] Evaluate dose response and PK/PD on reduction/reversal of sulfonylurea-induced insulin secretion (pharmacologic model of disease) [30-60 mg] CRINETICS PHARMACEUTICALS | 14 Healthy volunteers received single oral dose of CRN04777 (n = 9, 6 active / 3 placebo in each cohort) Baseline (Day -2) Treated (Day 1) “Glucose Clamp” with continuously adjusted glucose infusion rate (GIR) IV glucose Run in t=-60 min +8h IV glucose 0h SU (po) ‘4777 (po) SU (po) Multiple blood draws to measure glucose & insulin; Monitor GIR Run in t=-60 min +8h 0h Multiple blood draws to measure glucose & insulin; Monitor GIR 2. Sulfonylurea (SU) Challenge “Glucose Clamp” with continuously adjusted glucose infusion rate (GIR)

Sulfonylurea Challenge Provided Disease Relevant Proof-of-Concept Sulfonylureas block KATP channels, inducing insulin secretion, mimicking most common and severe monogenic form of congenital HI with KATP channel inactivating mutations CRINETICS PHARMACEUTICALS | 15 The administration of sulfonylurea is a pharmacologic model of congenital HI Sulfonylurea Challenge Sulfonylurea induces endogenous insulin secretion Automated delivery of supplemental IV glucose maintains blood glucose in the normal range (glucose clamp) Glucose infusion rate (GIR) increases in proportion to insulin secretion SST5 receptor Inappropriate insulin secretion Depolarization Ca2+ channel Glucose GLUT2 ATP/ADP Glycolysis Amino acids Ca2+ KATP channel Glucose sensing Glucose metabolism Hypoglycemia X X X X Amino acid metabolism
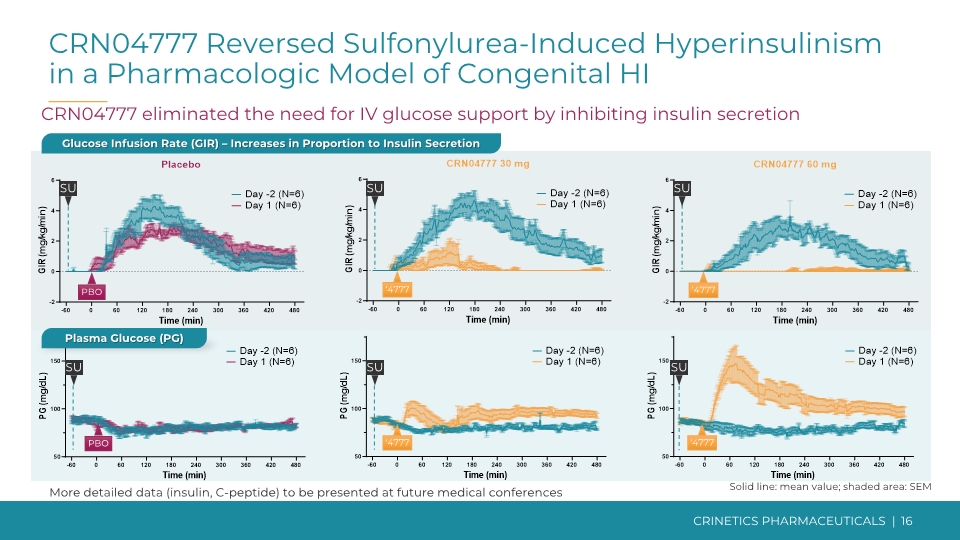
CRINETICS PHARMACEUTICALS | 16 More detailed data (insulin, C-peptide) to be presented at future medical conferences CRN04777 Reversed Sulfonylurea-Induced Hyperinsulinism in a Pharmacologic Model of Congenital HI CRN04777 eliminated the need for IV glucose support by inhibiting insulin secretion Plasma Glucose (PG) Glucose Infusion Rate (GIR) – Increases in Proportion to Insulin Secretion Solid line: mean value; shaded area: SEM
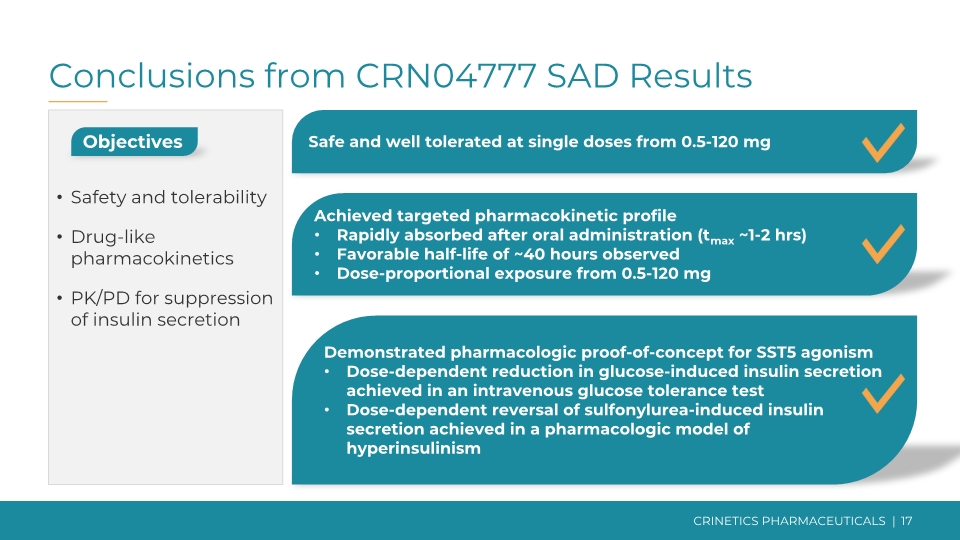
Conclusions from CRN04777 SAD Results Objectives Safety and tolerability Drug-like pharmacokinetics PK/PD for suppression of insulin secretion Safe and well tolerated at single doses from 0.5-120 mg Achieved targeted pharmacokinetic profile Rapidly absorbed after oral administration (tmax ~1-2 hrs) Favorable half-life of ~40 hours observed Dose-proportional exposure from 0.5-120 mg Demonstrated pharmacologic proof-of-concept for SST5 agonism Dose-dependent reduction in glucose-induced insulin secretion achieved in an intravenous glucose tolerance test Dose-dependent reversal of sulfonylurea-induced insulin secretion achieved in a pharmacologic model of hyperinsulinism CRINETICS PHARMACEUTICALS | 17

Pipeline With Three Candidates Beyond Pharmacologic Proof-of-Concept CRINETICS PHARMACEUTICALS | 18 Pharmacologic POC

CRINETICS PHARMACEUTICALS | 19 Achieved 2021 Goal of Three Programs with Clinical Proof-of-Concept CRINETICS PHARMACEUTICALS | 19 Executed development strategy with three NCEs: paltusotine, ‘4894, and ‘4777
